Moderna Vaccine California
California Suspends Use Of Moderna Vaccine Batch After Allergic Reactions. Moderna vaccine could be twice as effective as Pfizer against delta study finds Aidin Vaziri Rita Beamish Dominic Fracassa Anna Buchmann Aug.
Q A An Fda Panel Endorsed Moderna S Covid Vaccine One Ucsd Panelist Explains Why The San Diego Union Tribune
180 million Americans fully vaccinated Today during a White House briefing Jeff Zients the COVID-19 repose coordinator said 180 million Americans were fully vaccinated against COVID-19 as of yesterday.

Moderna vaccine california. Californias top epidemiologist told healthcare providers on Sunday to stop using a batch of Modernas COVID-19 vaccine after a higher than usual number of people had apparent allergic reactions. COVID in California. The Moderna COVID-19 Vaccine may not protect all vaccine recipients.
Moderna a Massachusetts-based vaccine developer partnered with the National Institutes of Health to develop and test a coronavirus vaccine known as mRNA-1273A clinical trial demonstrated that. The Moderna vaccine has been authorized by the FDA for use in people who are at least 18 years old. Of anaphylaxis in response to any COVD-19 vaccine not just Moderna.
Elasomeran codenamed mRNA-1273 and sold under the brand name Spikevax is a COVID-19 vaccine developed by American company Moderna the United States National Institute of Allergy and Infectious Diseases NIAID and the Biomedical Advanced Research and Development Authority BARDA. The California Department of Public Health has opened an investigation into one lot of a COVID vaccine from Moderna after it possibly. A health care worker fills a syringe with Moderna COVID-19 vaccine as California opens up vaccine eligibility to any residents 16 years and older during the outbreak of coronavirus disease COVID.
Pfizer and BioNTechs vaccine is authorized by the Food and Drug Administration for emergency use in people ages 16 and older. Moderna submitted its Biologics License Application to the FDA about one month after Pfizers submission meaning that full approval of its vaccine could be coming up within the next few weeks. After seeing what a friend who got the virus went through she got both doses of the Moderna vaccine.
Moderna says its COVID-19 vaccine candidate is 945 effective. Last month Pfizer boosted. As of Dec.
But almost immediately after the second. 19 there have been six cases in the US. Modernas vaccine has been shown in trials to be very effectivepreventing COVID-19 in 941 of the people enrolled in the Phase 3 clinical trial.
Medical experts such as Dr. The FDA has updated the the emergency use authorizations EUAs for both the Pfizer and Moderna vaccines to allow for. Pfizer is also expected to ask the.
This vaccine has a 941 efficacy rate can be stored at refrigerated temperatures and does not need to be diluted before it is given. January 16 2021 55 People Have Died in US After Receiving COVID-19 Vaccines. Though the headline crows about Hopeful Results in the Moderna COVID vaccine trials by the end of the paper we learn that 21 of people receiving the vaccine experienced a serious event.
Moderas vaccine is authorized for emergency use in those 18 and older. SAN FRANCISCO KGO -- Over the past few weeks California has rapidly expanded its vaccine eligibility meaning millions of people are getting a dose of the COVID-19 vaccine. Her friend recovered but five months after her second Moderna shot Kirkland remains in constant pain.
Overview of the Moderna Pediatric COVID-19 Vaccine Study. Again recipients of the Moderna vaccine followed by Pfizer then JJ had higher anti-spike Immunoglobulin G levels a sign of a stronger antibody response. Pfizer says its vaccine candidate co-developed with BioNTech is 95 effective.
California pauses use of Moderna vaccine batch over allergic reactions California health officials are asking to pause the use of a huge batch of Modernas coronavirus vaccine due to its higher-than-usual number of possible allergic reactions. Modernas views for total sales of its COVID-19 vaccine pale in comparison to the Pfizer-BioNTech partnership which has a higher manufacturing capacity for its shot. Anthony Fauci of The National Institutes of Health say that the Moderna vaccines mRNA components would have a similar success rate to the Pfizer vaccine which had an.
Additionally the vaccine seems to have broad efficacy with success rates consistent across various age race ethnicity and gender groups. Moderna filed for final approval of its vaccine on June 1 and expects to complete its submission in August. Kirkland 79 said she was healthy before she took the vaccine.
The vaccine maker is launching a clinical trial to assess safety of its vaccine in pregnant women despite 133000 pregnant women having already received a COVID vaccine. The CDC also tracked almost 4000 healthcare personnel first responders and. It is authorized for use in people.
In that time period more than 272000 people received the vaccine. Fact checkCOVID-19 vaccine recipients can donate plasma with the American Red Cross A substance called SM-102 is part of the Moderna vaccine but its not remotely what these posts claim it is. See Media Release here.
Rady Childrens Health Network is partnering with the Altman Clinical and Translational Research Institute and the Mother-Child-Adolescent Program at UC San Diego to study the safety and effectiveness of Modernas COVID-19 vaccine for children. Moderna says its vaccine is 93 effective against symptomatic illness at six months though this data collection ended before delta emerged in the US. The administration of over 330000 doses of Modernas covid vaccine were halted in California as health officials.
Adverse reactions reported in a clinical trial following administration of the Moderna COVID-19 Vaccine include pain at the injection site fatigue headache myalgia arthralgia chills nauseavomiting axillary swellingtenderness fever swelling at the injection site and erythema at the injection site. The Moderna COVID19 vaccine pINN. Experts Warn of Huge Risk as Moderna Launches COVID Vaccine Trials for Pregnant Women.
Kirkland did get the vaccine.

Us Has Enough Covid 19 Vaccines For Boosters Kids Shots The Financial Express
Oregon Moves Ahead With Moderna Vaccine After California Pauses Batch
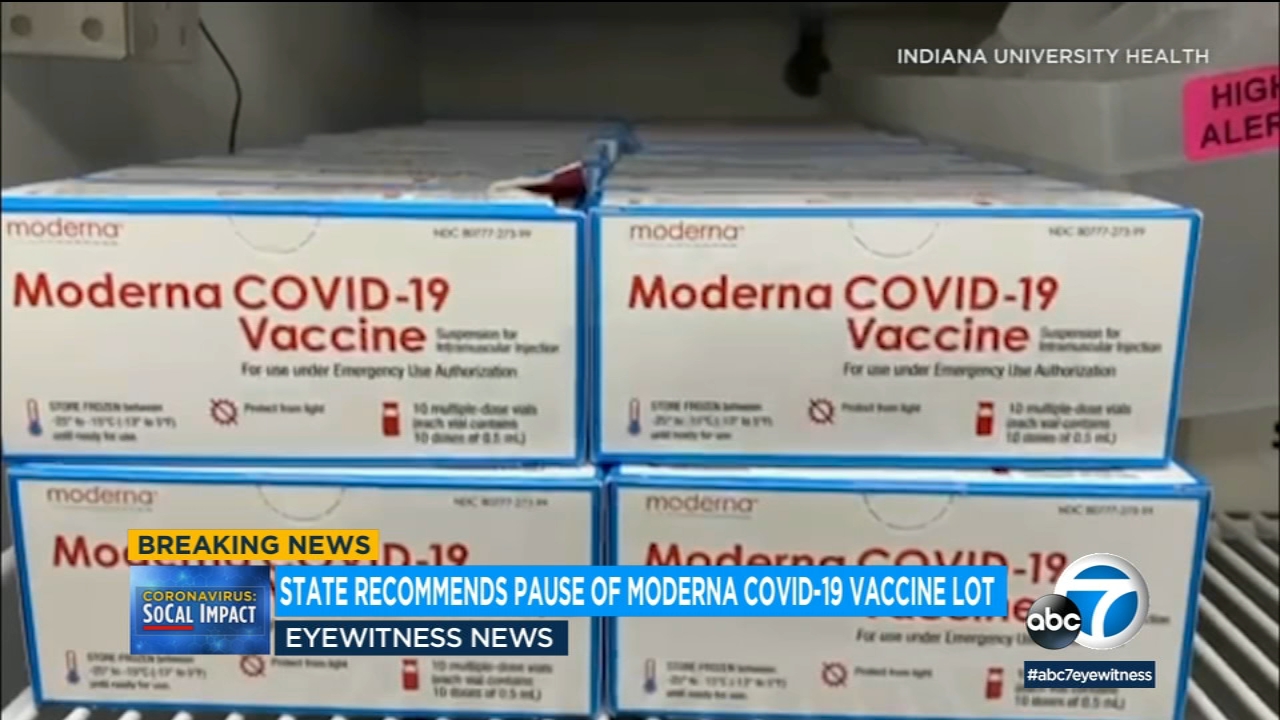
California Calls For Pausing Distribution Of Specific Lot Of Moderna Vaccine After Allergic Reactions Reported Abc7 New York
/cloudfront-us-east-2.images.arcpublishing.com/reuters/SU2QKHGSLVPEHIBIMJUA4ONZRA.jpg)
Moderna Plans To Expand Covid 19 Vaccine Production Wsj Reuters

Who Says Pregnant Women Should Not Get Moderna Vaccine The Times Of Israel

Ca Public Health On Twitter The Ingredients In The Covid 19 Vaccines Are No Mystery Read What S In The Moderna Pfizer And Johnson Johnson Vaccines Https T Co Le318hxhgk

5 San Francisco Bay Area Counties Say Providers Received Moderna Vaccine Lot Paused For Allergic Reaction Investigation Abc7 San Francisco

State Recommends Providers Pause Administration Of Specific Moderna Covid 19 Vaccine Lot Following Possible Allergic Reactions Yourcentralvalley Com
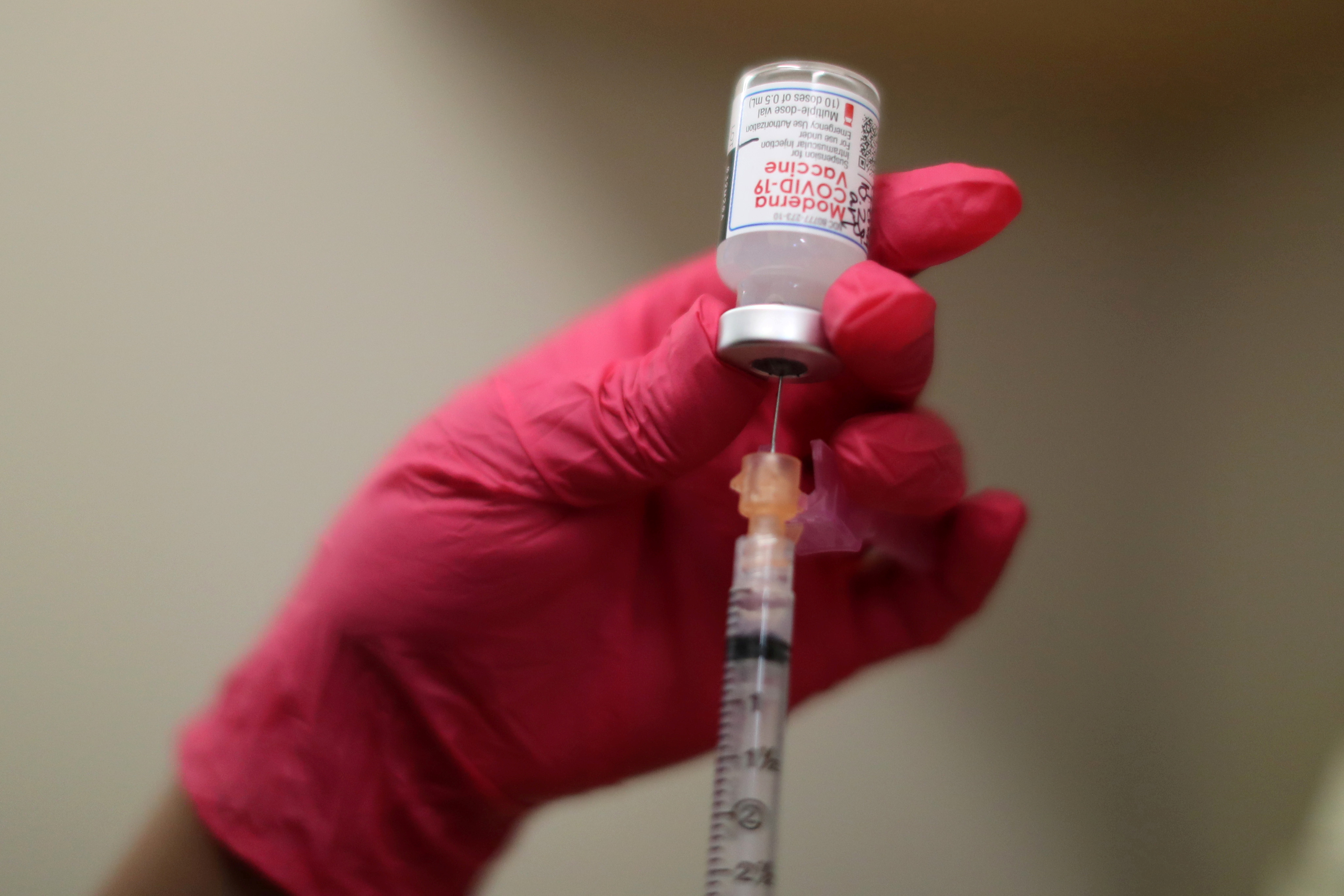
Moderna Reaches Supply Deal With Philippines For 13 Mln Vaccine Doses Reuters

Oregon Moves Ahead With Moderna Vaccine After California Pauses Batch

Drive Through Covid 19 Mass Vaccination Clinic Scheduled For Saturday In Salinas Monterey Herald

California Calls For Pausing Distribution Of Specific Lot Of Moderna Vaccine After Allergic Reactions Reported Abc7 New York

California Ok To Use Moderna Vaccine After Illness Reports Kpbs

California Woman Gets Injected With Wrong Vaccine For 2nd Shot Doctor Explains Impact Abc7 Los Angeles
Riverside County Albertsons Stores Temporarily Stop Accepting Appointments For Covid 19 Vaccinations Kesq
Moderna Covid Vaccine Enters Indian Market Pfizer Next Says Govt Latest News India Hindustan Times

Here S What Healthcare Workers Do With Extra Covid 19 Vaccines At The End Of The Day Abc4 Utah

Moderna Vaccine Protects Against Covid For Months Company Says Los Angeles Times



Posting Komentar untuk "Moderna Vaccine California"